Hydrogen for high-pressure gas quenching (HPGQ) has the highest thermal conductivity and heat capacity of any commercially available gas, but currently is not used due to safety concerns in commercial high pressure cooling applications. Somewhere, however, in a secret laboratory it receives research activity. A truly inert gas, helium is the next best choice, with a very good combination of thermal conductivity and heat capacity (specific heat, Cp). Nitrogen is a distant third, as indicated in the following chart:
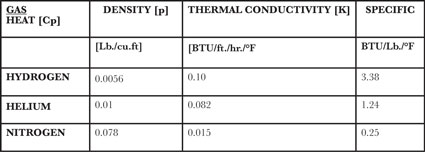
Helium’s ability to transfer heat away from a part is 5.5 times greater than nitrogen, and once heat energy is in the gas helium can hold five times as much. In either case, however, if the velocity of gas passing over the part is not uniform or does not exceed the thermal conductivity of the steel, the rate of heat removal will not be equal over the gear and the martensitic transformation will occur at different times, resulting in distortion and likely NMTP.
Nitrogen’s density is 7.8 times that of helium. Translated: fan horsepower (HP) for nitrogen must be 7.8 times higher, and then add 20-bar pressure (290 psia, 275.5 psig), and the horsepower difference is huge. Fan motor HP increases proportionally with pressure (gas density), and increases by the cube of the difference in RPM; double the RPM and the HP goes up eight times. Since gas velocity is also critical, fan output (RPM) must be high to transport heated gas to the water-cooled heat exchanger and back to the load. Even for helium, to move the quantity of gas through the load sufficient to quench alloyed steel gears, almost 400 horsepower is required. Employing the same HP for heavier nitrogen necessitates lower recirculation volume (smaller fan ,or lower RPM) to stay within 400 HP. And since nitrogen already has significantly lower heat transfer properties, combined with lower gas recirculation volume one can see its limitation. Consequently, only very high-alloyed steels—and thus more expensive—or certainly smaller cross-section gears qualify for nitrogen quenching.
The downside to using helium is the cost. At $25.00 per 100 cu. ft. it must be reclaimed, while nitrogen is only about $0.50 per 100 cu. ft. and can be thrown away after each cycle. Say you want to harden a 2-inch cross-section AISI 5130H gears, here’s how to determine if you can:
1) Go online and type “Timken practical data for metallurgists” and download the pdf file;
2) Go to page 56 and note the box containing “H value,” “quench,” and “agitation”;
3) 20-bar helium quenching, not shown, falls in at about 0.30 similar to very low agitated oil;
4) On the same page find the 2” diameter bar or one that comes closest to your part or gear cross-section;
5) Find the surface, ½ radius or center line and interpolate where 0.30 intersects that line and read down to the corresponding Jominy distance shown;
6) 2” bar at H = 0.30 intersects the “center” line at about 13.5 Jominy distance;
7) Now go to page 47 and locate 5130H steel. There the max./min. hardness is shown for the Jominy distance 13.5 is 20.5 to 34.5 Rc;
8) If your core hardness requirement is 32 Rc, then you likely can’t use the standard 5130 H steel but must specify a restricted hardenability (RH) chemistry that results in a Jominy hardness range no lower than 32 Rc. Final hardness is lowered by tempering;
9) If nitrogen is your HPGQ gas of choice it has an approximate H value of slightly less than 0.20;
10) Using the same 5130H Jominy table the surface of the 2” gear would barely make the core hardness using helium gas. This will force you to upgrade to a much more expensive alloy.
Having gone through the above, it will become obvious to those skilled in the art that in many instances the core hardness of the gear cross-section may not be the critical parameter, but the core of a tooth. Here two locations are important: tooth center on the pitch diameter (PD) and midpoint on a line drawn from root-to-root. Only empirical testing will confirm HPGQ suitability for any material. And when a suitable material is selected, a trial and error test plan including part location within the loading scheme will be required.





















