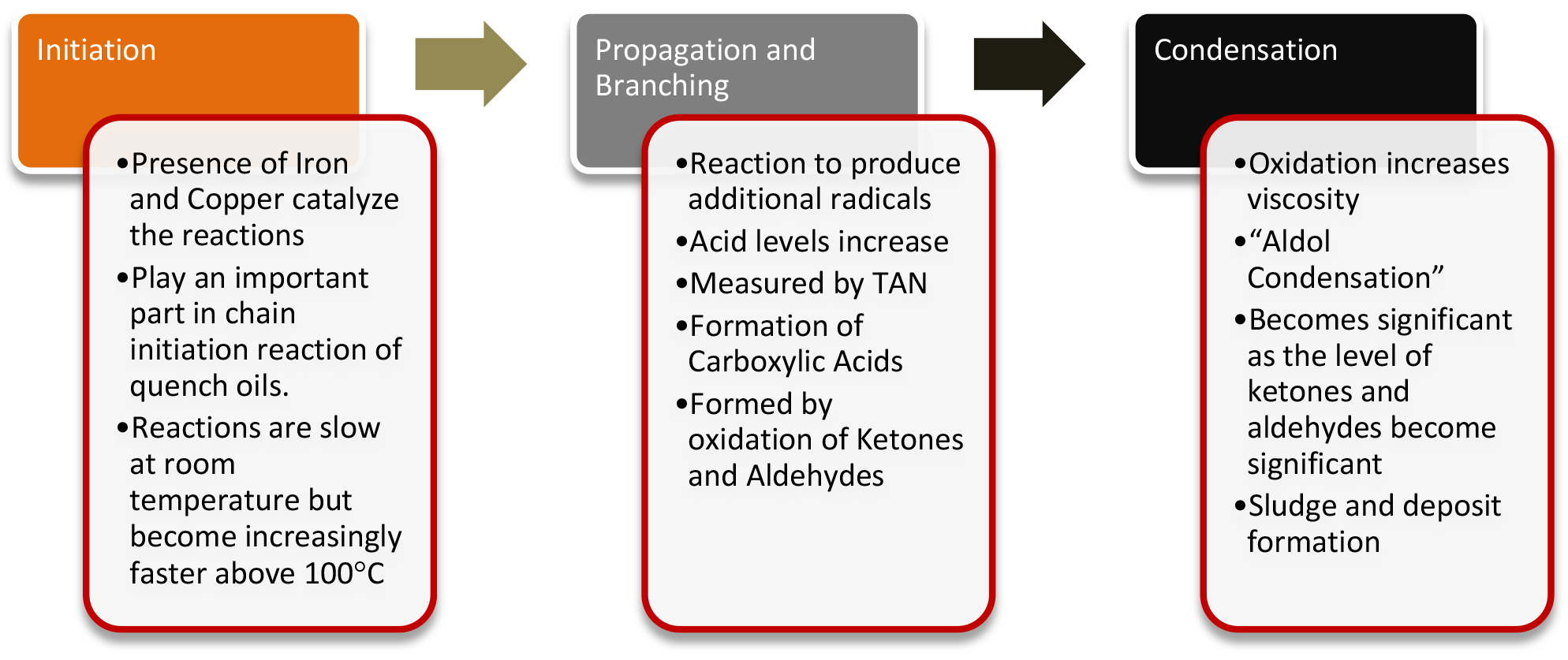
In the past several columns, the various components of a quench oil have been described. Illustrations on how the various components, such as the speed improvers, anti-oxidant packages, and base oil work together to reduce the oxidation of the oil, and provide long life and consistent quenching. In this column, typical causes and corrective actions regarding minimizing oxidation and sludge build up will be described. As a review, the mechanism of oxidation is shown in Figure 1. Oxidation of quench oils is aggravated by residues on parts from coolants, washer residues, excessive peak temperatures, high watt density heaters, and copper used for carburizing stop-off for carburizing. Oxidation of the oil is exhibited by elevated viscosity of the oil; higher quench rates, and part staining or “tiger striping.” The production of sludge also accompanies part staining.
Oxidation of the quench oil is monitored by the Precipitation Number (ASTM D91), Total Acid Content (ASTM D664), and Sludge content of the oil (ASTM D893).
Temperature is considered to be the greatest culprit in the oxidation of quench oil. One thing that is often neglected when designing a quench system is the energy density of the heating elements. If the heating element energy density is too high, the sheath temperature will be excessive, and the oil will rapidly oxidize by depleting the anti-oxidants present. The excessive sheath temperatures will also cause premature failure of the heating element. For most oils used for quenching, the recommended maximum energy density is 1-1.5 W/cm2 (5-10 W/in2). Higher energy densities may be used provided that adequate quenchant flow is provided around the heaters to minimize the local oil temperature.
It is also very important, should higher energy densities be utilized, that proper flow is maintained around the heater elements. If heat is to be maintained on the oil during weekend shut-down for instance, that the agitation is maintained to reduce the sheath temperatures. It is also recommended that the temperature be turned down to reduce time at temperature. The heaters should always be interlocked with the agitation system to prevent the heating elements from being energized when the agitation is off. However, regardless of the agitation, the sheath temperature should be limited to a maximum of approximately 50°C below the flash temperature of the oil. Agitation should be strong enough that a persistent vapor phase does not form around the heating element. Adequate replenishment of the quench oil through replacement of drag-out is important, as this addition of quench oil adds new anti-oxidants to the system. Do not allow the quench oil level to drop to avoid using quench oil. This is false economy, and will hasten oil oxidation.
The maximum peak oil temperature and the operating oil temperature are very important. This is true not only to minimize oxidation, but also for safety. The rule of thumb “one pound of parts to one gallon of oil” is recommended based on the balance of peak oil temperatures and life of the quench oil. The maximum operating temperature of the quench oil is based on the flash temperature of the oil. For example, a typical cold oil has a flash temperature (ASTM D92 Cleveland Open Cup) of approximately 350°F (176°C). The “one pound of parts to one gallon of oil” will produce approximately a 70°F (39°C) temperature rise. The NFPA, in its “Standard for Ovens and Furnaces” recommends a safety margin of 50°F (28°C) above the maximum peak temperature. As a quench oil supplier, we recommend an additional 50°F safety margin to further increase the safety margin for hung loads, high density packing, improved oxidation resistance, etc. These recommended temperatures are subtracted from the flash temperature to establish the recommended operating range of the quench oil. In this example, the maximum operating temperature of the oil would be 180°F (82°C).
Cooling of the quench oil, while not associated with oxidation, is necessary. It is recommended that cooling be accomplished by the use of air to oil heat exchangers. Water in quench oil can lead to disastrous consequences, such as fire and explosion. Water is to be eliminated from quench oil at all costs.

The Precipitation Number (ASTM D91) involves taking a 10 ml sample of the quench oil and mixing it with 90 ml of a suitable solvent in a cone shaped centrifuge tube. Hexane is usually used. This mixture is then centrifuged. After centrifuging, the volume of the solid sediment at the bottom of the tube is read and reported (Figure 2).
In the case of Figure 2, an oil showing severe blackening of parts was tested and found to be severely degraded. With this much sludge, it is likely that there is considerable amount of sludge at the bottom of the quench tank. For most normal testing, a limit of 0.5 percent is considered a high precipitation number.
The Total Acid Number test (ASTM D664) determines the acid content of a quench oil sample by a simple titration. The TAN is expressed as the mg of KOH (potassium hydroxide) necessary to neutralize a gram of oil sample. This can be accomplished either by a color change of an indicator or a potentiometric method.
As the additive package in an oil is depleted, the oxidation process creates acid, which in turn, produces oxidation products. The TAN measures the amount of acid produced by the oxidation process. Contamination by hydraulic fluids such as phosphate esters can elevate the TAN number and produce false readings. Confirmation by FTIR can verify if the elevated TAN is due to oxidation or contamination by other sources.
There are four different ASTM methods for determining the TAN of a sample. The numbers obtained by each of the methods may not give the same result, so it is incumbent on the user to make sure that the same method is used to determine TAN. A TAN greater than
1.5 – 2.0 mg KOH/g strongly suggests that staining of a part will occur. A new oil will have a TAN of typically less than 0.3 mg KOH/g.
When the precipitation number is high and the TAN is elevated (greater than 2), it indicates that the oil has considerable amount of oxidation products present. These oxidation products, besides staining parts and producing sludge, can also coat the surfaces of heat exchangers and reduce the efficiency of the heat exchangers. This results in longer cycle times to cool the oil. Further, if the quench tank has considerable sludge, then the available volume of quench oil is reduced, increasing the peak temperatures. The sludge present can also block baffles and reduce available agitation. This can cause distortion or poor properties.
If the precipitation number is high, but the TAN is low, then this suggests that contamination from some source has occurred. During carburizing, soot formation is likely with poorly adjusted generators, or with too aggressive boost-diffuse cycles. This soot gets carried into the quench oil with the result that part staining results, with the staining looking identical to the staining from oxidation.
Once an oil is badly contaminated, or has a high TAN or precipitation number, it is often sent out for filtration. The oil can be filtered onsite by a service or the oil can be filtered by trucking the oil away. The cost of this process is expensive — usually about half the cost of new oil. The oil should be tested after filtration and prior to entering into service. This is to make sure that the cooling curve compares favorably with new oil, and if any additive package is necessary to bring the oil back to near new condition. The oil should be tested for water to make sure that a new hazard isn’t introduced into the system.
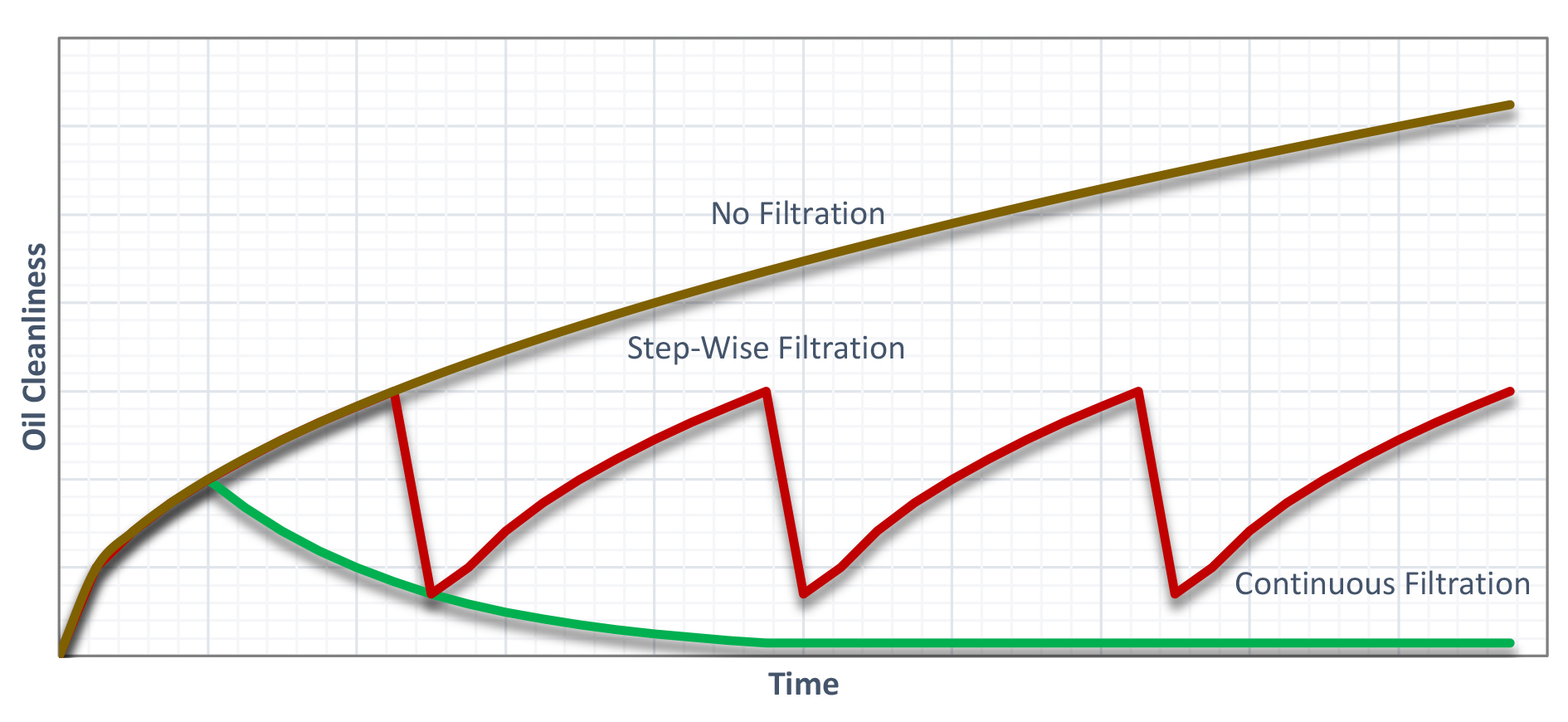
However, this method produces a saw-tooth pattern of oil cleanliness. It is better, and more cost effective from a maintenance and production standpoint to filter continuously. This can be seen from Figure 3.
It is also appropriate to start filtration when the oil is new. If filtration is started once the oil is already dirty, then the filter media will clog rapidly, resulting in short media life and frustrated maintenance. The filtration should be continuous. The goal is not to clean to a minimum level and let the oil become dirty again, but to maintain the oil in “like new” condition. It is in this condition that the oil will last the longest.
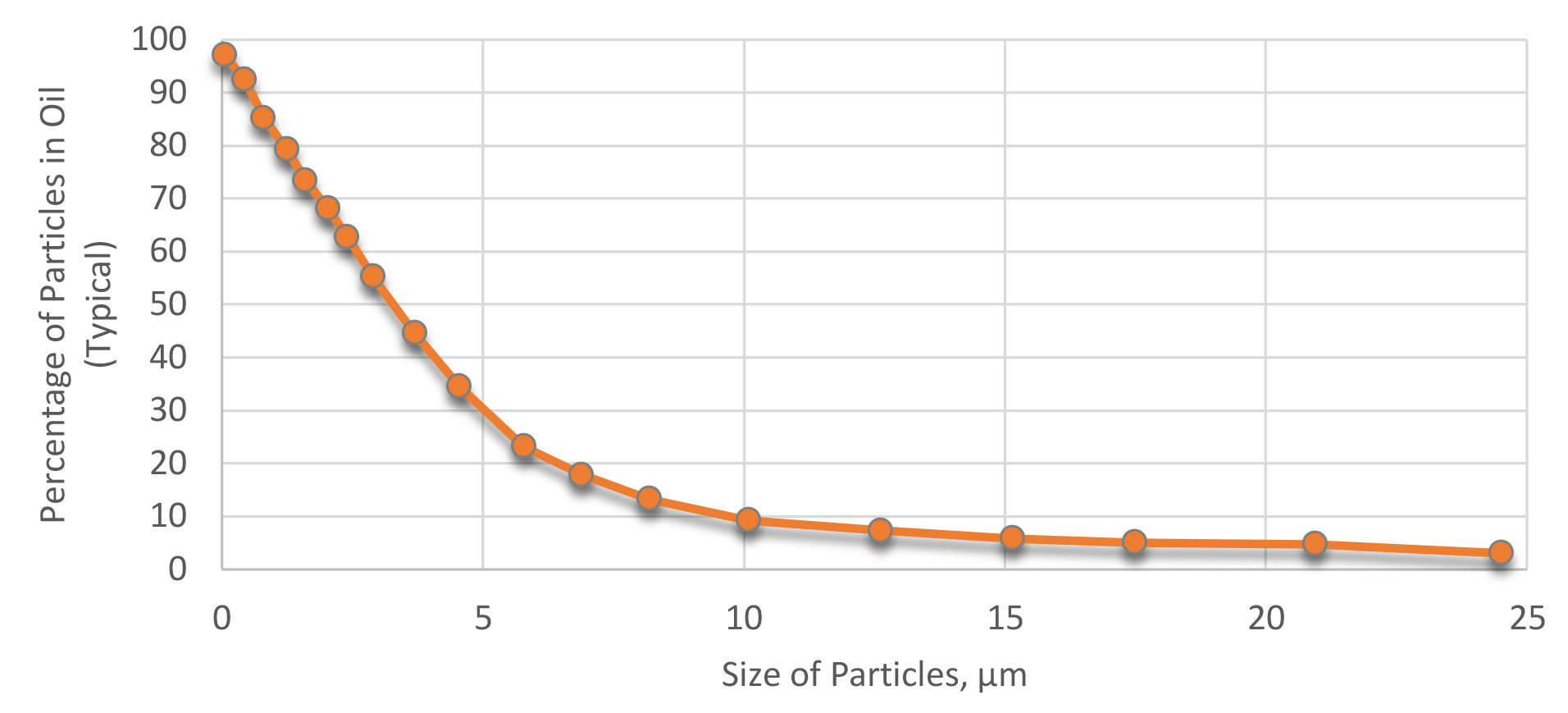
A good filtration system should capture all the contamination (sludge, soot, scale, and water) found in a typical quench system. It should be capable of filtration to at least 5µm. While many people filter to 20µm, this is only capturing a small amount of the total dirt load (Figure 4). The greatest percentage of particulate in quench oil is less 5µm. To achieve proper filtering, a filtration system is necessary that is capable of filtering to around 3µm or less, and is capable of capturing sludge, and has a high load capacity (Figure 5).

Summing up, oxidation of a quench oil is a serious problem that can be overcome, with appropriate corrective actions:
Ensure operating parameters are set up correctly, with appropriate interlocks to prevent the heaters from being energized if agitation is not enabled.
Ensure heating elements are the appropriate energy density for the oil. An energy density of 6-10 W/in2 (1-1.5 W/cm2) is recommended.
Load density is maintained at the “one pound of parts and racks to one gallon of quench oil” rule of thumb.
Filtration of oil using high capacity continuous filtration can significantly prolong and reduce oxidation of the quench oil by removing precursors of oxidation. Improved part cleanliness will result.
About the author D. Scott MacKenzie, Ph.D., FASM, is senior research scientist-metallurgy at Houghton International Inc. For more information, go to www.houghtonintl.com.






















