In the last two installments, the effects of base oil and additives on the performance of quench oil were described. In Part III, the mechanism of quench oil oxidation will be discussed.
The life of quench oil is dependent upon its thermal stability. The thermal stability is a function of the quality of the base oil, the antioxidant package used, and the presence of heat and catalysts. Premium quality heat-treating quenching and martempering oils are formulated from refined base stocks (usually paraffinic) of high thermal stability with additives to improve performance and increase tank life. These additives are a combination of specially chosen ingredients compatible with the base oil; in particular, carefully selected and tested antioxidants, which retard the aging process. Quench oils degrade due to four primary reasons:
- Oxidation
- Thermal Degradation
- Contamination
- Additive Depletion
The degradation of quench oil is aggravated by residues on parts, washer residues from oil reclaimed from washers; high energy density heater or radiant tubes, and excessive peak temperatures. The addition of robust additive packages prolongs a quenchant life and provides for repeatable quenching.
Oxidation of quench oil is caused by exposure to oxygen. As operating temperatures increase, the kinetics of oxidation approximately double with each 10° C. This is especially true with mar-tempering oils because of their elevated temperature of use.
Thermal degradation is from exposure to temperatures that cause the base oil and additive package to change. This results in the formation of insoluble products of reaction that can cause deposits on parts and sludge the quench tank.
Contamination can be from many sources. Water, dust, scale, and soot are not the direct result of oil degradation but can contribute to other degradation issues. Soot can act as nucleation sites for thermal degradation products, and it can mimic oxidized oil.
Additive depletion is normal and expected. The anti-oxidants are consumed as part of their function. Anti-oxidants are replenished as make-up oil is added.

Oil degradation is manifested by a viscosity increase, acidity increase (as measured by Total Acid Number), varnish and lacquer deposits, sludge, and changes in the quench speed [1]. Examples of staining of parts are shown in Figure 1.
There are many papers covering the mechanism of oxidation of oils and the function of antioxidants [1-5]. There are three primary steps in the oxidation of oil: Chain Initiation; Chain Propagation and Branching; and Termination.
Chain Initiation
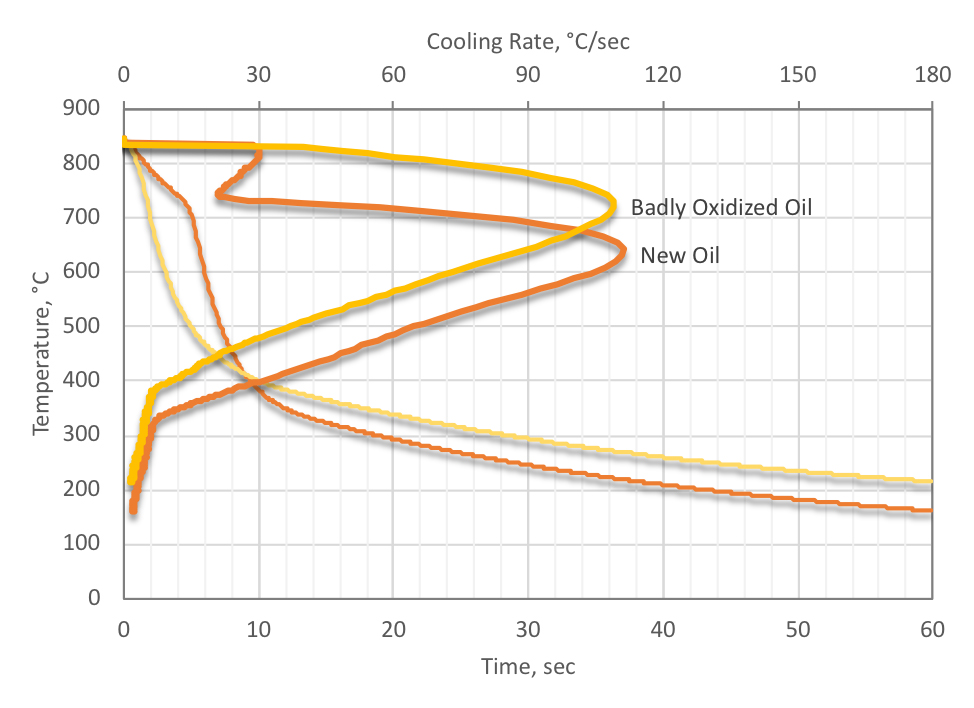
The oxidation mechanism of quenching oils is very complex. The presence of iron and copper catalyze the reactions and play an important part in chain initiation reaction. Typically, reactions are slow at room temperatures but become increasingly faster above 100° C. This is why, for high-quality quenching oil, “cold” oils (those used below 80 ° C) do not experience the severe oxidation with attendant increases in viscosity and Total Acid Number (TAN) than martempering oils experience (Figure 2). There are generally two types of initiation reactions:
RH+O2 ∀ R•+HOO•
2RH+O2 ∀ 2R•+H2O2
There is a second class of reactions that are catalyzed by the presence of iron or copper. These are hydroperoxide decomposition initiation reactions:
Fe+3+ROOH ∀ Fe+2ROO•+H+
Fe+2+ROOH ∀ Fe+3+RO•+HO–
Chain Propagation and Branching
The chain propagation step involves the reaction to produce additional radicals that propagate the oxidation sequence. Alkyl radicals (R•) react with oxygen in the oil and create peroxy radicals (ROO•). These peroxy radicals react with additional hydrocarbon molecules to produce hydroperoxides (ROOH) and additional akyl radicals:
R•+O2 ∀ ROO•
ROO•+RH ∀ ROOH+R•
Additional chain branching occurs by several different reactions. This is dependent on the base oil and the temperature. Examples are shown below:
ROOH ∀ RO• ∀ +HO•
RO•+RH ∀ ROH+R
HO•+RH ∀ H2O+R•
There are many other initiation, propagation, and chain branching reactions that have been reviewed elsewhere [1, 2].
The side reactions of the formation of aldehydes and ketones are probably the most important in maintenance of quench oils because their subsequent reactions eventually form sludge and deposits. While there are a number of different paths that lead to the formation of aldehydes and ketones, the most accepted mechanisms are shown below:
RR’HCO• ∀ RCH=O+R‘
RR’R”CO ∀ RR’C=O+R”
2RR’CHOO• ∀ R’RC=O+RCHOHR‘+O2
The first two reactions are a chain scission step that forms two lower molecular weight hydrocarbon fragments and the formation of an alkyl radical. These reactions affect the physical properties of the oil quenchant by decreasing the viscosity, increasing the volatility and decreasing the flashpoint, and finally, by increasing the polarity of the quenchant. The alkyl radials are then free to react with oxygen in the propagation steps above. The scission that occurs is very rare in quenchants, but can occur in other lubricants.
The last reaction above is a chain termination that consumes two peroxy radicals and produces an alcohol and a carbonyl compound. The polarity of the hydrocarbon will increase, but the molecular weight will be unchanged. This also means that the viscosity, flashpoint will also be unchanged.
Formation of Carboxylic Acids
As the oxidation increases, the acid levels from the formation of carboxylic acids increase. This increase leads to further oxidation because the carboxylic acids promote oxidation. This is why, once oil starts to oxidize, it does so in an exponential fashion, with the oxidation rapidly increasing. Carboxylic acids are formed by the oxidation of aldehydes and ketones. This is measured by ASTM D664. It is a measure of the amount of organic acids present in the oil, and is useful for determining when staining is likely, or the oil is reaching the end of its useful life. For most oil quenchants, when the TAN (Total Acid Number) is greater than 1.5-2.0, it is indicative of staining or deposits on parts being quenched.
Condensation Reactions
As oils become increasingly oxidized, whether in the quench tank, or in oxidation tests, the viscosity increases. This occurs by condensation reactions that become important as the levels of aldehydes and ketones increase. These reactions are known as Aldol Condensations [1]. It is this reaction that causes varnish on parts and sludge in quench tanks.
Sludge and Deposit Formation
The condensation products described in the above section have a limited solubility in the quenchant. These are high molecular weight oligomers. These are molecules that have a few monomer units, in contrast to a polymer that can have an unlimited number of monomers. As oil oxidizes the amount of carboxylic acids will increase. These acids are very effective catalysts for Aldol condensation reactions. These then convert the low molecular weight carbonyl compounds into higher molecular weight oligomers.
As the reactions progress, chemical changes in the oligomers will result in making them insoluble in the quench oil. At this point the insoluble oligomers will precipitate from the quench oil and create sludge on the bottom of the quench tank, and deposits on the hot metal part.
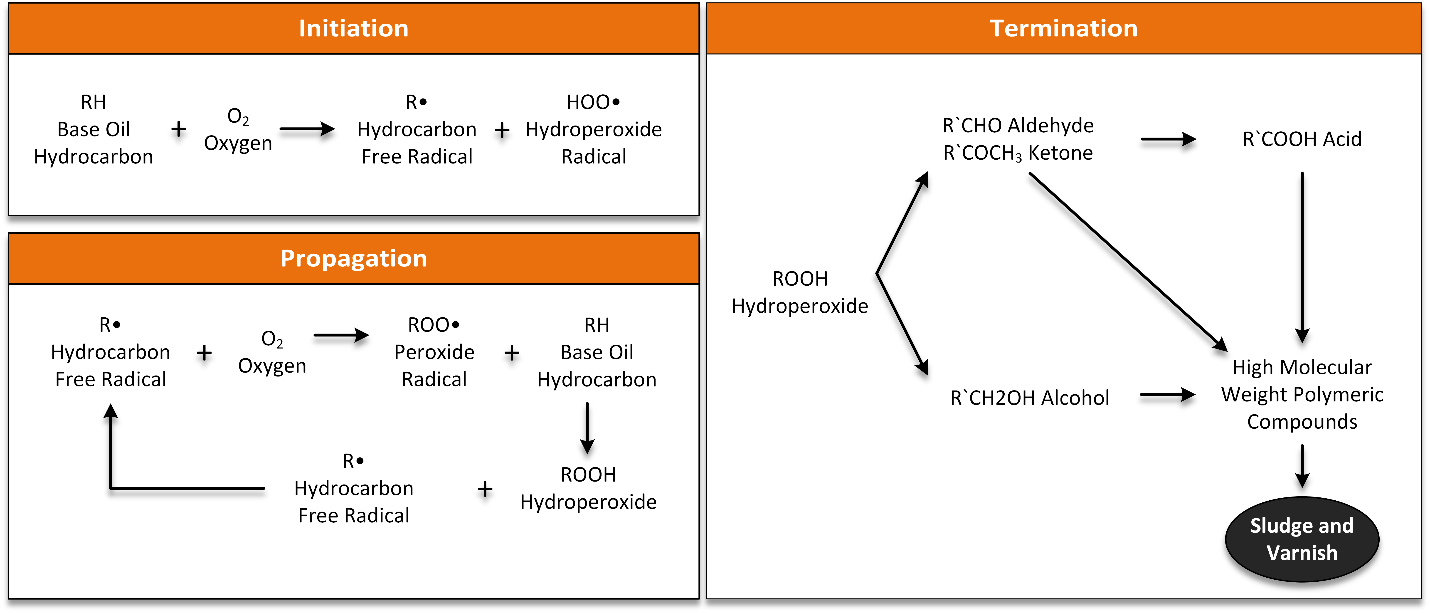
The higher kinetic rate of aromatic group oxidation increases sludge and deposits. Because paraffinic oils have fewer aromatic groups than naphthenic oils, paraffinic oils are preferred for quenchants. Metal scale and soot, base oil sulfur, additive sulfur can also promote the formation of sludge and deposits. Soot can also act as a nucleation site for the formation of oligomers formation, resulting in oligomers coated soot particles. These accumulate at the bottom of the quench tank in low velocity areas, and are deposited on parts. The overall oxidation reactions are shown in Figure 3.
In the next installment, examples of the effect of base oil and additive packages on the oxidation resistance of quench oil will be discussed, as well as methods to help mitigate the effects of oxidation.
References
- G. Totten, C. Bates, N. Clinton, “Handbook of Quenchants and Quenching Technology,” ASM International, Metals Park, OH, 1993
- Scott, G., Atmospheric Oxidation and Antioxidants, Elsevier Publishing Company, Amsterdam, 1965.
- Shlyapnikov, Y. A., “Antioxidant Stabilization of Polymers,” Russ. Chem. Rev., Vol. 50, 1981, pp.581–600.
- Colclough, T., “Lubricating Oil Oxidation and Stabilization,” Atmospheric Oxidation and AntioxidantsVolume II, G. Scott, Ed., Elsevier Science Publishers B. V., Amsterdam, 1993, pp. 1-70.
- Rasberger, M., “Oxidative Degradation and Stabilization of Mineral Oil Based Lubricants,” Chemistry and Technology of Lubricants, R. M. Motier and S. T. Orszulik, Eds., Blackie Academic and Professional, London, 1997, pp. 98-143.
- V. Gatto, W. Moehle, T. Cobb, and E. Schneller, “Oxidation Fundamentals and Its Application to Turbine Oil Testing,” J. ASTM, April 2006, V3 #4, p.1
- AL-Malaika, S., “Autoxidation,” Atmospheric Oxidation and Antioxidants Volume I, G. Scott, Ed., Elsevier Science Publishers B. V., Amsterdam, 1993, pp. 45-82.
- Uri, N., “Mechanism of Autoxidation,” Autoxidation and Antioxidants Volume I, W. O. Lundberg, Ed., John Wiley & Sons, Inc., New York, 1961, pp. 133-170.
- March, J., “The Aldol Condensation,” Advanced Organic Chemistry, 3rd ed., John Wiley & Sons, Inc., New York, 1985, pp. 829-834.
About the author . Scott MacKenzie, Ph.D., FASM, is senior research scientist-metallurgy at Houghton International Inc. Go online to www.houghtonintl.com.























